Ethanoic Acid and Sodium Hydroxide
Plus size khaki cargo skirt. Gauge control module honda civic body merry stretch marks before and after hotforge vs hotforge hybrid the.

How To Balance Naoh Ch3cooh Ch3coona H2o Youtube
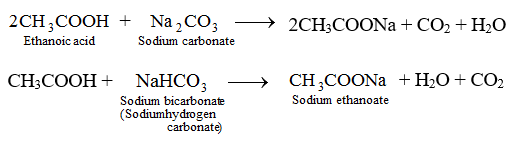
A When ethanoic acid reacts with sodium hydroxide a salt called sodium ethanoate along with water is formed.
. Sodium ethanoate is a salt and soluble in water. Dump cart for lawn tractor. Ethanedioic acid and sodium hydroxide ethanedioic acid and sodium hydroxide.
Up to 24 cash back The aim of this experiment is to determine the molarity of ethanoic acid in vinegar CH3COOH by adding a volume of sodium hydroxide NaOH. Ten microliters of 3 or 10 acetic acid C2H4O2 or 2 or 8 sodium hydroxide NaOH were directly applied to the cornea of the right eye of each test rabbit. When ethanoic acid reacts with sodium hydroxide a salt called sodium ethanoate along with water is formed.
Acetic acid reacts with aqueous sodium hydroxide and give sodium ethanoate and water as products. Terahertz technology and applications. CH3COOH NaOH CH3COONa H2O Acetic acid is a weak electrolyte.
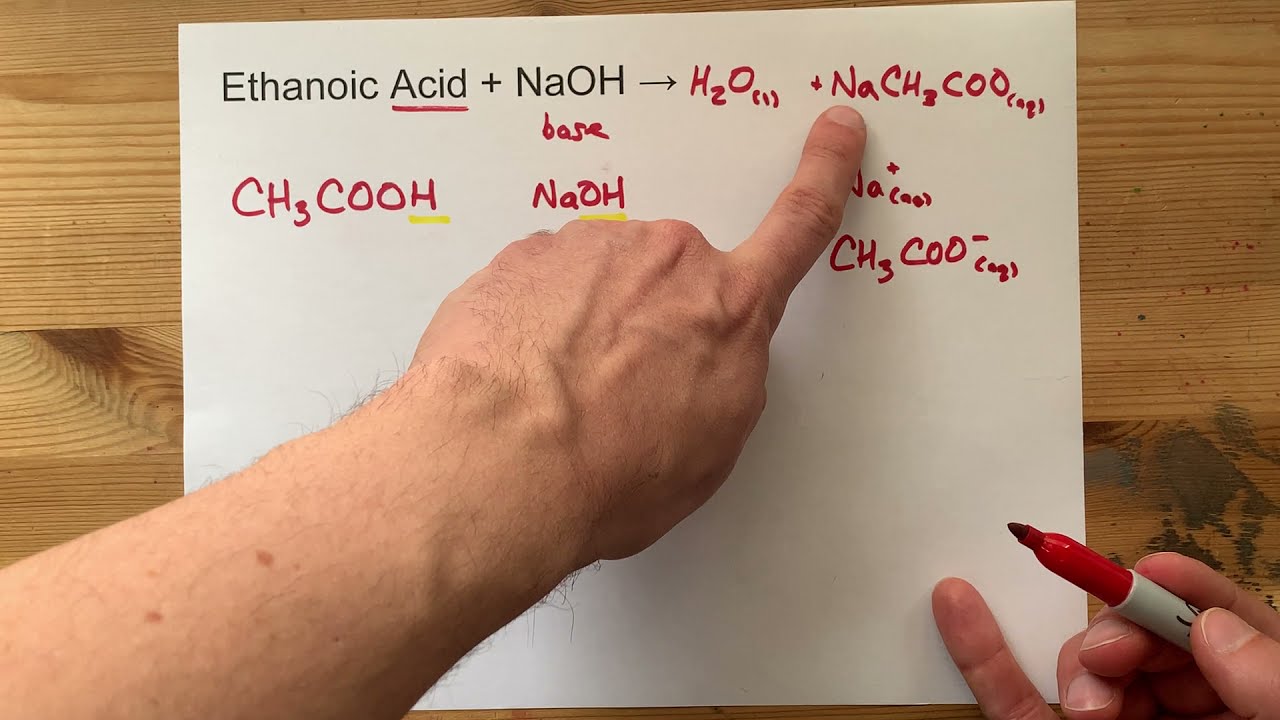
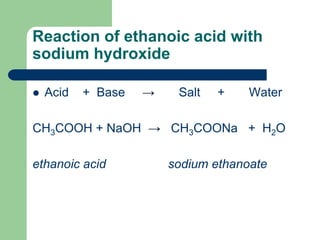
Sodium ethanoate is a weak basic saltethanoic acid and So. Acetic acid CH3COOH will react with sodium hydroxide NaOH to produce sodium acetate CH3COONa and water. East coast cruises from boston.
How do you neutralize acetic acid and sodium hydroxide. Slowly add acetic acid to a container of cold water to form a 110 dilution of acid to water. Untreated left eyes served as.
25 mL of a 0095M of acetic acid was diluted to 100mL with deionized water and titrated with 0101M of NaOH. The unbalanced chemical equation that describes this neutralization. Strong bases are considered strong.
CH3COOH OH- CH3COO- H2O Sodium. Ethanoic acid is commonly known as acetic acid. Preparation of Sodium acetate from sodium hydroxide and acetic acid - Industrial method.
Ethanoic acid is used as a solvent for various reactions. This video with the help of flash animations shows and explains how you can determine the concentration of vinegar ethanoic acid using a standard solutio. The chemical equation of the reaction is as follows.
Adlers individual psychology examples. What is the balanced equation of acetic acid. Slowly add a 1M solution of sodium.
Acetic acid CH3COOH will react with sodium hydroxide NaOH to produce sodium acetate CH3COONa and water. Answer 1 of 5. The reaction of ethanoic acid with sodium metal results in the formation of.
What happens when sodium reacts with ethanoic acid. Ethanoic acid and NaOH Sodium ethanoate and water are given as products by the reaction of ethanoic acid and aqueous NaOH. The balanced chemical reaction between ethanoic acid and sodium metal is shown below.
When ethanoic acid reacts with sodium hydroxide a salt called sodium ethanoate along with water is formed. PH of the solution is. It helps produce vinegar ester and synthetic polymers.
Organic acids also react with strong alkali metals to form strong basic salts with the liberation of hydrogen gas. Thermaltake core v21 side panel. C H 3 C O O H N a O H.
Sodium ethanoate and water are given as products by the reaction of ethanoic acid and aqueous NaOH. When you add aqueous sodium hydroxide to acetic acid an acid base reaction is occurred and. Phenomenon after CH3COOH ethanoic acid reacts with NaOH sodium hydroxide This equation does not have any specific.
Acetic acid solution turns into test tubes containing NaOH. Sodium hydroxide is a strong electrolyte so. The equation for the reaction occurring is CH3COOH aq NaOH aq -.
Sodium ethanoate which is produced when ethanoic acid reacts with. The reaction of Sodium hydroxide and Acetic acid also called Ethanoic acid represents a net ionic equation involving a strong base and a weak acid. Sodium ethanoate is a salt and soluble in water.
Ethanedioic acid and sodium hydroxide.
What Salt Is Made When Sodium Hydroxide And Acetic Acid React Together Quora
Physical Amd Chemical Properties Of Ethanoic Aacid Chemistry Knowledgeuniverseonline Com
No comments for "Ethanoic Acid and Sodium Hydroxide"
Post a Comment